See how Clear Trials works.
We transform complex trial documents into plain-language resources that participants can access anytime. Here's what that looks like—for you and for them.
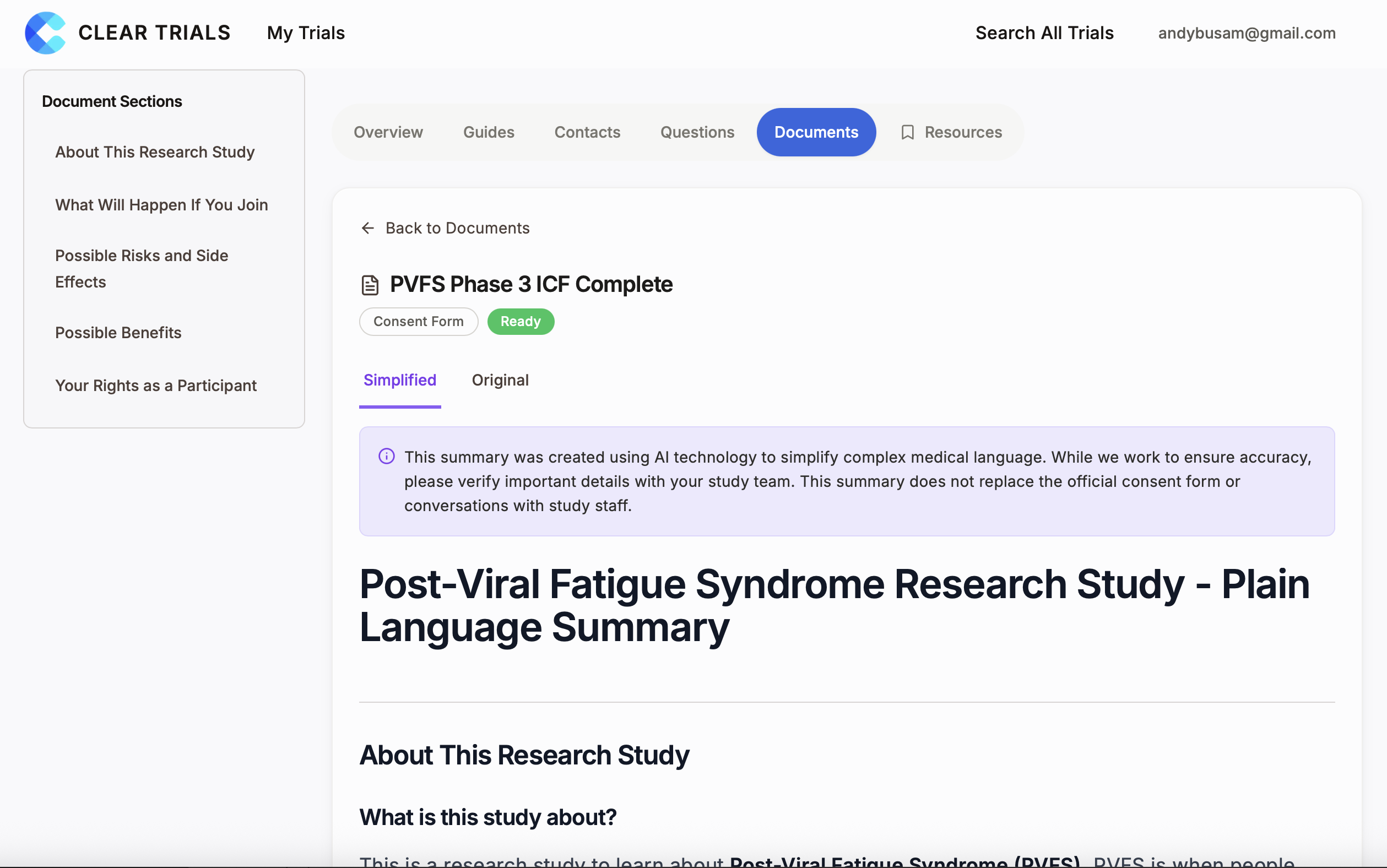
How it works for your organization.
Upload your documents
Share your IRB-approved consent forms, protocols, and participant instructions. We accept PDFs, Word docs, or direct integration with your document management system.
We generate plain-language versions
Our AI-powered system—with guardrails fully vetted by IRB partners—transforms complex medical and legal language into clear explanations at a 6th-8th grade reading level. Unlike generic LLMs, our system operates within strict, contained boundaries with full audit trails. Every output is editable and fully reviewable by humans before participants ever see it.
You review and approve
Every simplified document goes through your review before participants ever see it. You can edit any content, review complete audit trails, and maintain full control over what gets published. Nothing reaches participants without your explicit approval.
Participants get access codes
Coordinators provide access codes at enrollment. Participants use them to log in and see their trial information—simplified, searchable, and always available.
What participants see.
When a participant logs in with their access code, they find everything about their trial in one place—explained in language they can actually understand.
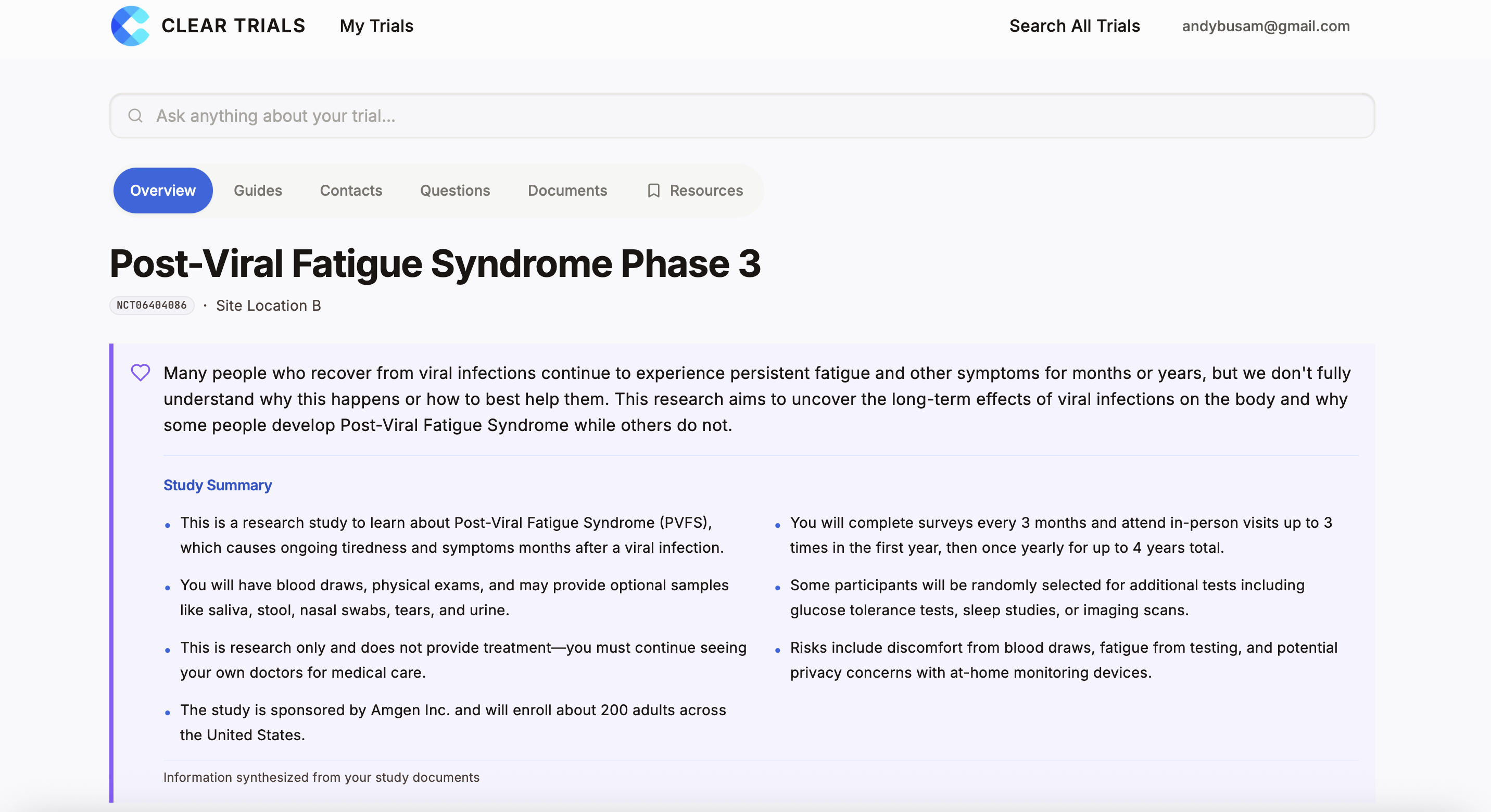
"My Trial" Dashboard
All documents and information in one view
Plain-Language Summary
Complex consent form explained clearly
Ask a Question
Get answers from their actual documents, not generic info
Share Access
Invite caregivers to see the same information
Everything participants need to stay informed.
AI-Powered Simplification with IRB-Vetted Guardrails
Complex consent forms and protocols transformed into clear, accessible language. Unlike generic LLMs, our system uses strict guardrails fully vetted by IRB partners. Every output is contained, editable, includes complete audit trails, and is fully reviewable by humans before sharing with participants.
Ask Questions
Participants type questions naturally—"Can I take ibuprofen?" or "What happens at my next visit?"—and get answers sourced directly from their trial documents. No hallucination risk.
Caregiver Sharing
Participants can invite family members or caregivers to access the same information. The people supporting them stay informed too.
24/7 Access
Questions don't wait for office hours. Participants access their trial information from any device, whenever they need it.
Document Source Attribution
Every answer links back to the official source document. Participants see exactly where information comes from, and are always directed to their study team for clinical questions.
Built for how you work.
Clear Trials integrates into your existing workflow without adding burden to coordinators or compliance risk to your organization.
You Stay in Control
Every simplified document is reviewed, editable, and includes complete audit trails. Your team approves everything before publication. Nothing reaches participants without your explicit sign-off.
Flexible Integration
Work with us however fits your workflow. Upload documents directly, connect via API, or let our team handle transformations as a managed service.
Compliance by Design
HIPAA-compliant infrastructure on AWS. ICH-GCP adherent. No PHI stored in our application database. Your regulatory team can review our security documentation anytime.
